The following solutions are fully worked through in a PDF that is available to print.
2. (a) Use the equation for the total energy available to the products:
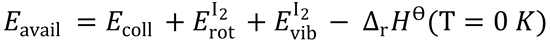
ΔrHθ = -170 kJ mol-1
-
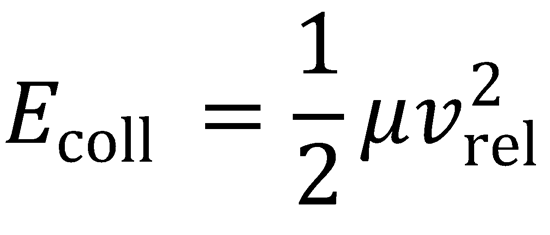
Ecoll = 10.8 kJ mol-1
-
Erot(I2) = RT (using the Equipartion principle for a linear molecule: each quadratic term in the
Hamiltonian contributes ½RT to the internal energy).
-
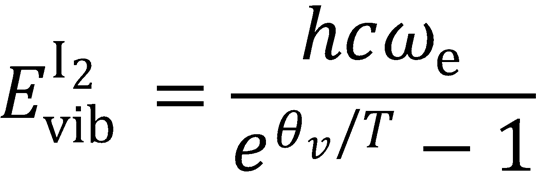 (the high temperature limit cannot be used here because only the lower vibrational
(the high temperature limit cannot be used here because only the lower vibrational
levels are populated).
Evib(I2) = 1.426 kJ mol-1
∴ Eavail = 10.8 + RT + 1.426 + 170 = 184.7 kJ mol-1
(b) |ℓmax| = μvrelbmax (1)
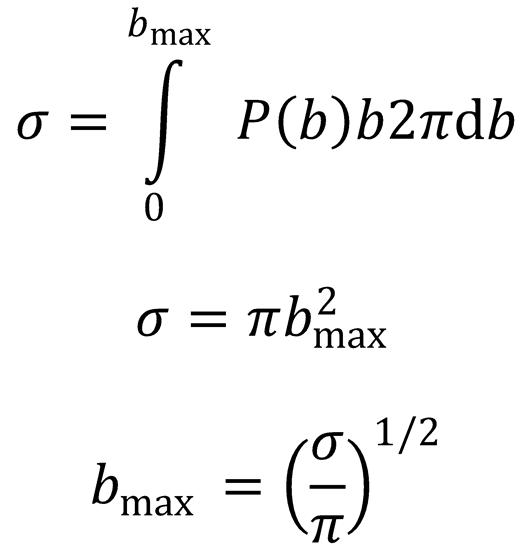
Substitute the expression for bmax into (1) to obtain a value for |ℓmax|:
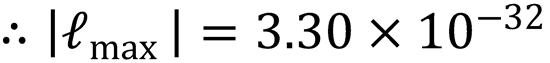
Using the fact that 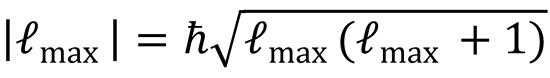 :
:
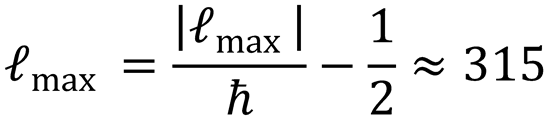
(c) j' = ℓmax ≈ 315
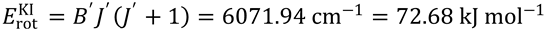
3. (a) Plot a graph to show consistency with the line-of-centres model:
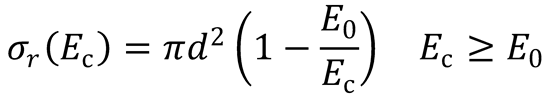
Rearranging the equation gives:
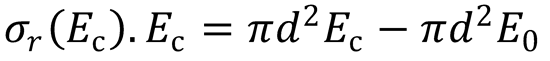
Therefore, plot a graph of σr(Ec).Ec vs Ec to produce a straight line;
gradient = 𝜋d2
y-intercept = – 𝜋d2E0
|
Ec/kJ mol-1 |
σr(Ec)/10-10 m2 |
σr(Ec).Ec/10-10 m2 kJ mol-1 |
|
9
|
0.5 |
4.5 |
|
15 |
1.25 |
18.75
|
|
30 |
2.0 |
60
|
|
50 |
2.2 |
110
|
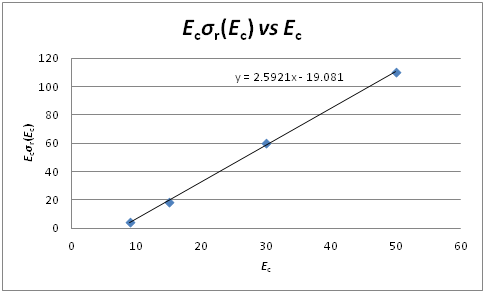
gradient = 𝜋d2 = 2.5921
∴ 𝜋d2 = 2.59 Å2
y-intercept = – 𝜋d2E0 = – 19.081
∴ E0 = 7.36 kJ mol-1
(b)
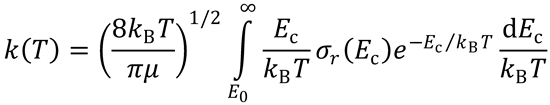
Let x = 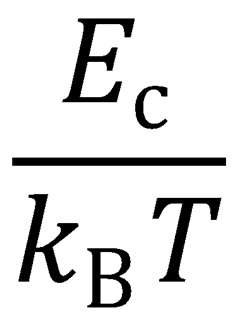
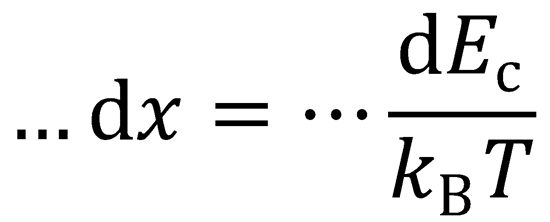
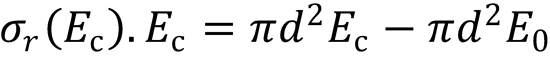
Ec = xkBT
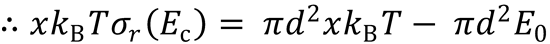
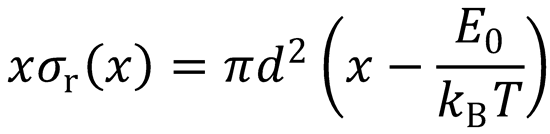
E0 → E0/kBT, so 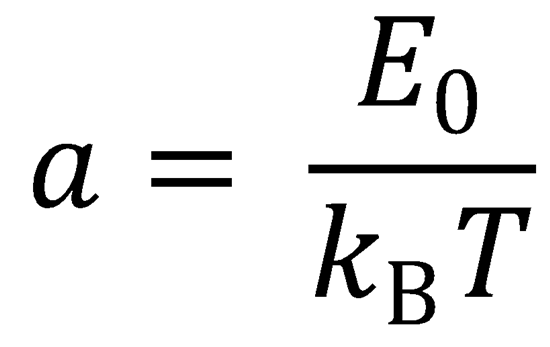
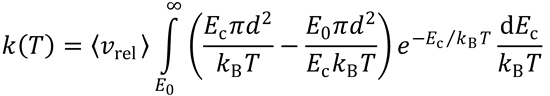
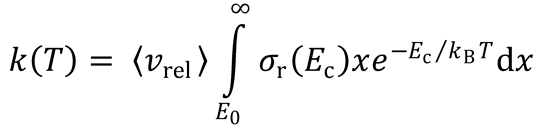
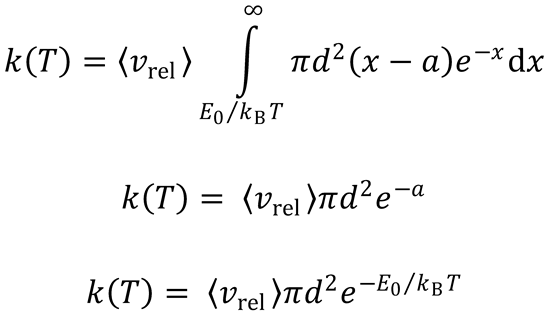
This is identical to the expression obtained for simple collision theory (SCT).
Return to Problem Set