1. The reaction C(3P) + C2H2 at low collision energies proceed via an addition-elimination mechanism whereby
formation of an intermediate, C3H2, which lives many (>10) rotational periods, is followed by dissociation to
C3H + H products. Knowing also that the C3H radical product is highly rotationally excited, what do you expect
the angular distribution of the C3H product to look like in the CM reference frame?
2. At low collision energies the reaction O(3P) + H2S → HSO + H proceeds via a direct mechanism on the triplet
potential energy surface, while the corresponding reaction of excited oxygen atoms O(1D) + H2S → HSO + H
proceeds via a long-lived complex, thyo-peroxide HSOH, on the singlet potential energy surface. What do you
expect the angular distribution of the HSO product in the CM-frame of reference to look like in the two cases?
3. With reference to the reaction O(1D) + H2S → HSO + H, what experiments would you perform to estimate the
lifetime of the HSOH complex within the osculating complex model for chemical reaction? Knowing that at a
given collision energy the average lifetime of the HSOH complex is equal to its rotational period, what will be
the ratio of the backward to forward intensity of the HSO product CM angular distribution?
4. The figures below show the KI product flux contour plots in the centre-of-mass (CM) frame for the K + I2
(left) and K + CH3I (right) reactions.
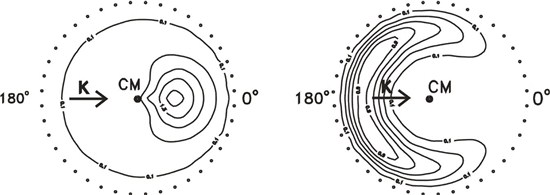
Explain how data like these might be obtained.
What can be learnt about the dynamics of the two reactions from these figures? (The outer rings of dots show
the maximum CM velocities of KI in the two reactions.)
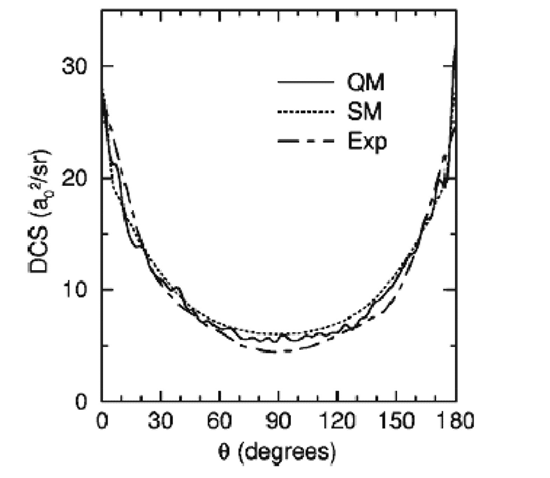
Contrast the scattering behaviour observed for the above two reactions with that found for the reaction
S(1D) + H2 → SH + H, the differential cross section for which is shown below [adapted from E. J. Rackham,
T. Gonzalez-Lezana and D. E. Manolopoulos, J. Chem. Phys., 2003, 119, 12895].
5. As we have seen in this Chapter, OH in its ground electronic state undergoes the reaction
OH + D2 → HOD + D,
which is isoelectronic with the F + H2 reaction. Figure 6.24 shows plots of the D-atom product flux in the
centre-of-mass frame for the above reaction. The experiments were performed under crossed molecular
beam conditions, with the D-atom products detected by the H(D)-atom Rydberg tagging method. The dashed
rings are labelled according to the number of quanta m in the bending mode, and n in the OD stretching mode
of HOD (m, n).
(a) What does the above figure suggest about the mechanism of the reaction?
(b) The reaction has also been studied in crossed molecular beam experiments using mass spectrometric
detection of the HOD product. Using an appropriate Newton (velocity-vector) diagram for the HOD
products, suggest how the product flux contour map of HOD might differ from the one shown in Figure
6.24 for the D atom products. Assume that the reactant beam velocities are the same in the two different
experiments.
(c) What spectroscopic experiments might be performed to probe the transition state region of this reaction
(or a similar reaction) more directly? (It might be helpful to reread Section 1.4.5, and also refs. [14] and
[542] concerning the anion photoelectron detachment spectroscopy of [FH2]- and [H3O]-.)
Above Problems are available as a PDF to print
Solutions to Chapter 6 Problems