The following solutions are fully worked through in a PDF that is available to print.
1. (a) Kinetic energy = ½mv2
Use the expression: eV = ½mv2
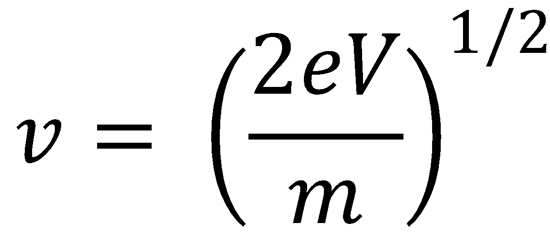
∴ v = 128 600 ms-1
The distance the ions fly is d = 40 cm = 0.4 m.
Flight time, t = d/v
∴ t = 3.11 µs
(b) The radial distance travelled by the atoms in 3.11 µs = 12.68/2 = 6.34 mm.
v = d/t
∴ v = 2038.59 ms-1
(c) Conservation of energy requires that:
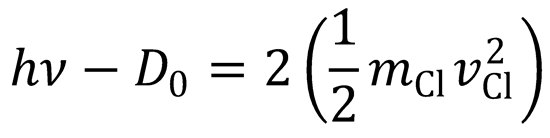
D0 = 243 kJ mol-1 = 4.035 x10-19 J.
mCl = 5.812 x 10-26 kg
vCl = 2038.59 ms-1
h𝜈 = 4.035 x 10-19 + 2(½ x 5.812 x 10-26 x 20389.592)
∴ h𝜈 = 6.449 x 10-19 J = 4.026 eV
To calculate the wavelength:
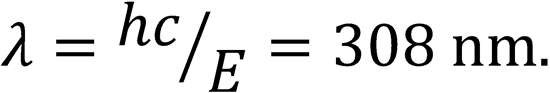
Return to Problem Set