1. Velocity-map imaging was used to detect Cl fragments from the photodissociation of molecular chlorine after
they had travelled along a 40 cm flight path from the interaction region to the detector. The resulting image is
shown below.

(a) A potential of 3000 V is used to direct the ionized Cl atoms to the detector. What is their flight time? Take
the mass of a Cl atom to be 35 g mol-1.
(b) The image appears as a single ring of Cl atoms as a result of conservation of energy and momentum. The
outside diameter of the ring is 12.68 mm. What velocity did the Cl atoms acquire as a result of the photo-
dissociation?
(c) The bond dissociation energy of Cl2 is 243 kJ mol-1. Use conservation of energy to determine the
photolysis laser wavelength.
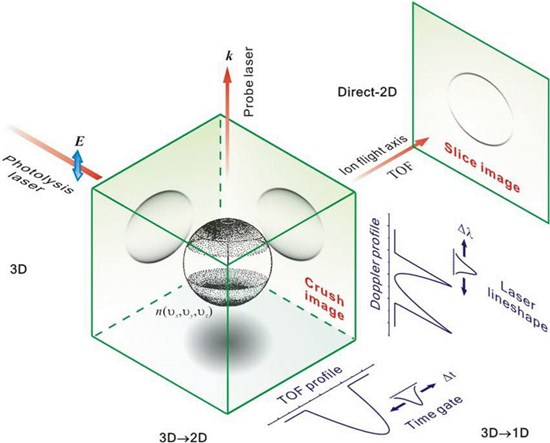
2. The figure below compares an ion imaging, Doppler profile, and TOF measurement of a Newton sphere with
β = +2. The power of 2-D ion imaging is in measuring slow velocities, especially compared to equivalent
1-D techniques.
(a) Figure 8.10 of this chapter shows the velocity distribution of Br atoms produced in the 510 nm photolysis
of Br2. Use the velocity corresponding to the peak in the distribution to calculate the laser bandwidth at
which one would begin to resolve the 1-D Doppler profile for the Br atoms. In these experiments, Br is
detected by (2 + 1) REMPI at 235 nm.
(b) Photoionization of Br by (2 + 1) REMPI at 235 nm creates Br+ + e- at 2.5 eV excess kinetic energy.
Calculate the recoil velocity (in ms-1) imparted by the ionization, and compare it to the Br velocity
resulting from the photolysis process, shown in Figure 8.10.
(c) What velocity resolution might one obtain using the original ion imaging method, in which the ~2 mm
beam diameter is also projected onto the detector? Assume in your calculation that the 'ring' in the Br
image has radius 10 mm.
3. (a) When HCl, jet-cooled in a molecular beam, is photodissociated at a wavelength of 210 nm, H atoms are
observed with two different speeds corresponding to total kinetic energy release (TKER) of H (2S) + Cl(2P)
atoms of 10989 cm-1 and 11871 cm-1. The ratio of signal intensities for the slower to faster H atoms is
0.75. Both photodissociation channels show anisotropy parameters of β = -1. Use these data to deduce
the bond dissociation energy (D0) of HCl, and account for the observations of faster and slower channels.
What non-adiabatic dynamics must be occurring in the HCl molecule during dissociation? Use the ratio of
signal intensities for the two channels to estimate the probability of a non-adiabatic transition, assuming
that the state initially populated in the photoexcitation correlates with spin-orbit ground state products.
(b) If HI is photodissociated at 258 nm, product H atoms are again observed, but with kinetic energies of
6525 cm-1 and 14128 cm-1. The faster H atoms again have β = -1 but, for the slower H atoms, β = +2.
Derive a value of D0 for HI and explain the observed values of the anisotropy parameter.
[Hint: It might be helpful to take another look at Sections 4.2.4, 4.2.6, and 8.8.6 before attempting this
question.]
Above Problems are available as a PDF to print
Solutions to Chapter 8 Problems